Of all the types of products known to humanity, sterile products attract the closest scrutiny from the authorities. Mainly, this is because any errors in the preparation could be fatal to the users of these substances. Yet old methods of preparing sterile products continue to be in use. Aseptic processing is strict because lots of undetected microbial impurities find their way into the pharmaceutical content. Isolated systems have been implemented overtime to make sure that there is no room for the penetration of pathogens.
The sterility concept

source: healthline.com
In microbiological terms, there are only two kinds of products the sterile and non-sterile. Most products on the market today are non-sterile, and they include tablets creams, oral syrups, and medical liquids and gases, as well as ointments. On the other hand, non-sterile products such as infusions and injections form the lower side of the percentile. In the past, injections did not necessarily require sterilization, and therefore, there was no need for tests. Since then, unfortunate incidences have necessitated scrutiny and sterilization. The advancement of medical technology now allows for the administration of small antibodies to solve different illnesses. These products are sterile until they are injected into the body.
Ideally, a sterile product is free and clean from any pathogens or bacteria. But, in science, it is almost impossible to have 100% sterile liquid. So, statistics show the level of microorganisms in a product. Different industrial approaches have been used to test for still different products. The problem is that the organisms are not evenly distributed in a drug, and this means some parts might be cleaner than others. Sterility, therefore, becomes hard to test, especially on the spectrum of quality assurance.
Sterilization
source: pexels.com
The process of sterilization depends on the product and the manufacturer. For example, the terminal station involves the ceiling of a product in a container that is bacteria proof before being taken through the process. Heat in the form of steam, gases or radiation is directed to the product.
In the end, terminal sterilization removes these microorganisms to the chances of one in a million. However, traditional sterilization procedures are not the best because they may end up damaging the quality of reactive products. For instance, proteinous solutions could easily be lyophilized when exposed to heat or radiation. This is where aseptic preparation comes into play.
Aseptic preparation
source: medicalnewstoday.com
The main idea of aseptic preparation is to remove any chances of external contamination by microbial impurities. Therefore, the product is blended from already terminally sterilized ingredients. The conditions are set to prevent any impurifications. Therefore, theoretically, the SAL of aseptically prepared products is equal to the specific components that have been used in the process.
Such a situation would only stand if no organisms are impurities ingress. However, the process needs to be extremely tight. Aseptic preparation has several challenges because it requires perfection. Microbial ingress could be detected.
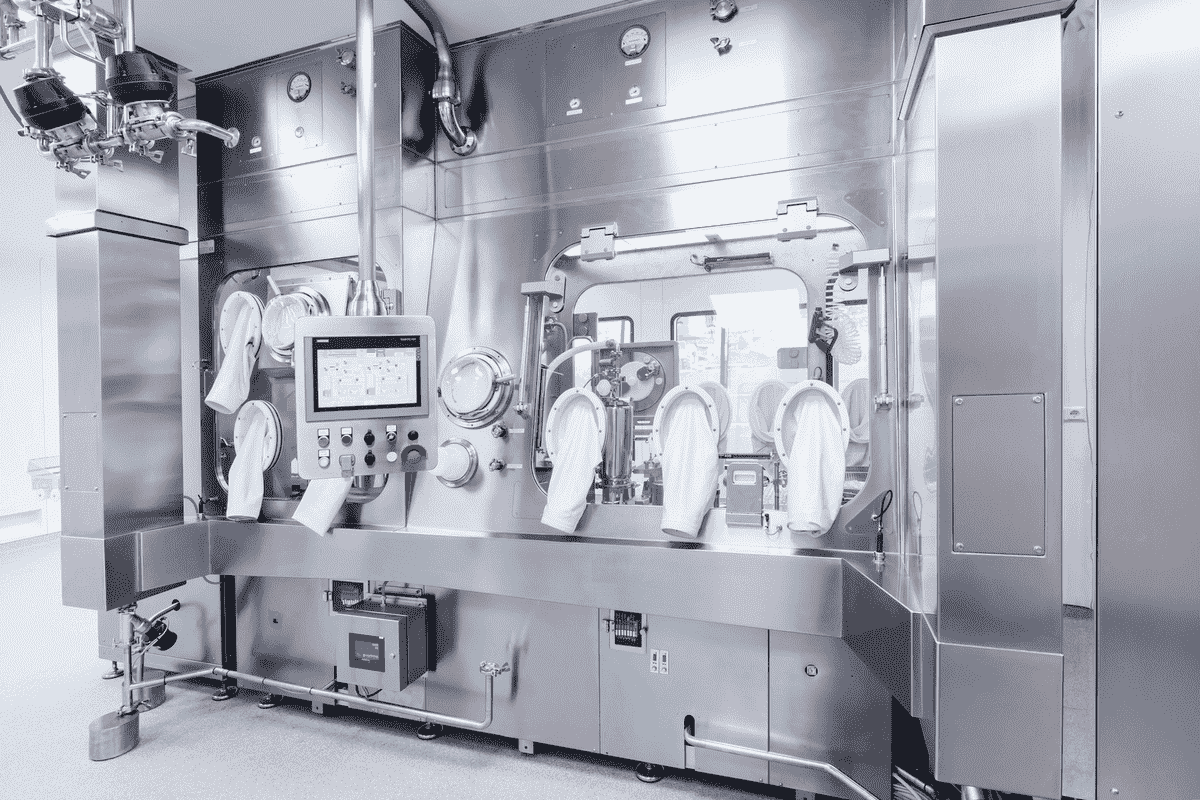
Managing the risks
source: thermofisher.com
Technology has shown that we can strengthen the weak links in aseptic preparation by providing high-quality pharmaceutical equipment and systems. By the 1960s, pharmacists already knew that they could maintain aseptic conditions by not exposing the products to air.
Again, the operators had to have the right gear and instructions. And, because the majority of microbial immigration comes from humans, there must be minimum interaction of the product and the operators. Below are some of the mitigation practices used to maintain aseptic preparation.
The environment
source: medicalnewstoday.com
Keeping the environment around the product clean can increase the chances of maintaining the ideal conditions for aseptic preparation. For example, the development of microelectronics that can clean air has been used in the Unidirectional Laminar Airflow (LAF) systems.
Already, you realize that the conditions needed to have higher standards, especially for aseptic processes, as opposed to those in terminal sterilization. This reflects the disparity of quality between the two products. Maintaining a clean environment goes to the extent of limiting the presence of operators during the process.
The operator
source: medicalnewstoday.com
There are several advances in technology that helps to clean air. But, the human potential of the operator is still constant without any upgrades. This means that the operator may need to improve their gear and attire as well as the accessories and equipment they use.
Also, they must receive advanced aseptic training to understand how the process works. Yet even after all these things have been done, operators still pose the most significant contamination in The LAF and clean rooms.
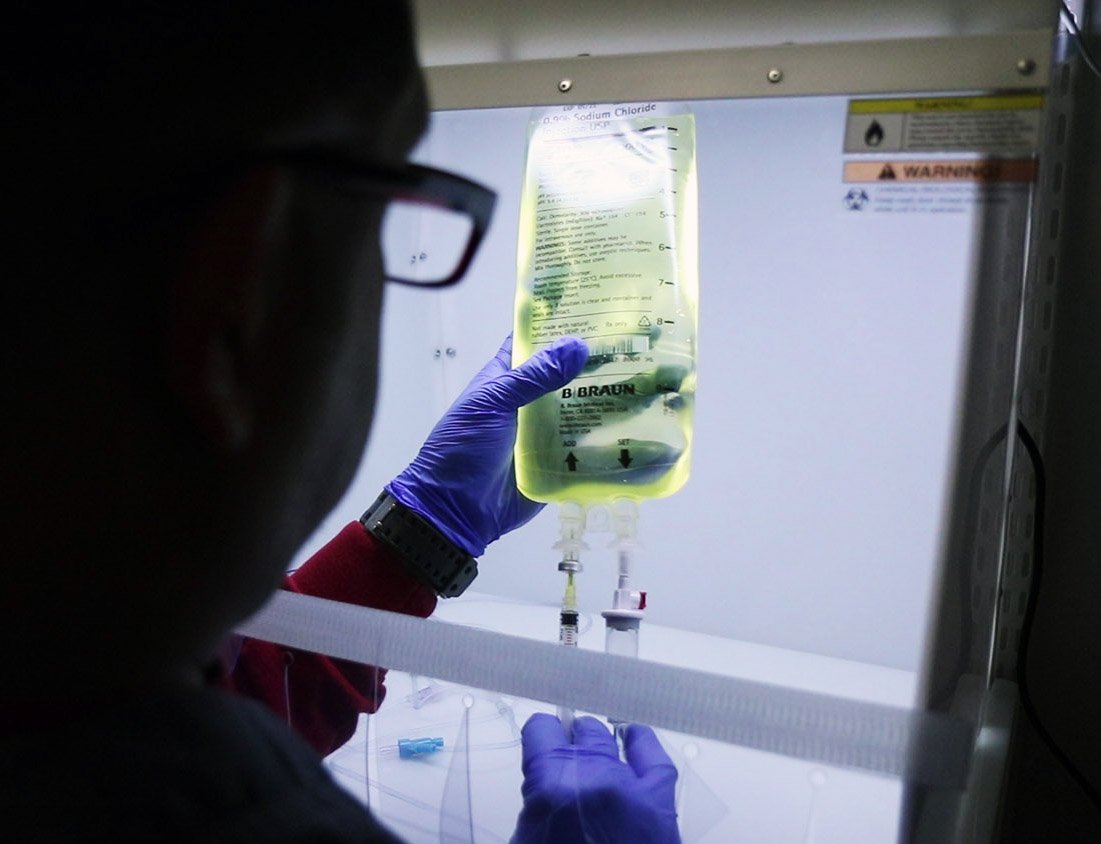
The isolators
source: sentryair.com
Another significant cause of contamination could be the systems being used by the manufacturers. As already mentioned, the aseptic preparation process needs to be perfect. Therefore, any contamination, whether from the operators or the environment or even the isolators, should be kept at bay. Isolators our systems put in place to ensure that substances that quickly react with the air are handled safely. In the same way, they put operators away to minimize contamination.
Since the 1940s, isolators have been used by nuclear operators as protection from radiation and harmful substances. But, today they are apart in their septic preparation to keep the content clean. The gloves are usually treated with vaporized hydrogen peroxide, which kills or microbial intruders.
You may also like to know about How to Prevent Heart Disease at Any Age
Steriline Aseptic Processing
source: healthcarepackaging.com
If you want to get top-notch quality, airtight aseptic equipment, you may want to try out Steriline brands. The company has been around for several decades now, offering both robotic and mechanical solutions that make aseptic preparation easy.
Their solutions cover toxic and nontoxic substances, including powders and liquids. The single-source manufacturer of pharmaceutical equipment boasts highly advanced solutions to equipment for injectable packaging products.